A data collection form must be completed and two (2) copies must be sent with the plasma sample to the Québec Antiretroviral Therapeutic Drug Monitoring laboratory. We suggest that you keep one copy for your records.
Click here to order requisition forms for your centre.
There are several components to the data collection form. However, all of the information requested is necessary to make the best interpretation of the concentrations and to provide the most adequate recommendations for the patient. We ask that the requisition be completed in its entirety as all of the information is essential to the individualization of the pharmacological advice which will accompany the results.
See below for an explanation of each element requested.
Please remember to indicate the following:
- Antiretroviral(s) to be analyzed
- Antiretroviral dose and schedule of administration (# of daily doses)
- Date and time of the last dose
- Date and time of the blood draw
- Previous virologic failure to protease inhibitors
- Cumulative list of protease mutations (or reverse transcriptase mutations if requesting etravirine TDM)
- Adherence data
Patient identification

We ask that you provide the patient identification information at the top right of the form (patient name, medical record number, date of birth, gender, health insurance number). This information is required for identification of the report that will be sent to the physician. The age of the patient will allow individualization of the interpretation, particularly in paediatrics. All information will remain confidential and will only be consulted by the personnel of the Québec Antiretroviral Therapeutic Drug Monitoring Program.
Physician identification
Complete mailing address for sending the interpretation report is necessary. Also, phone and fax numbers for crucial value reporting are essential. For administrative and legal reasons, the license number of the treating physician must be completed.
Patient information

Weight/height: Low body weight and obesity may potentially influence antiretroviral concentrations. Calculation of the body surface area is also essential in paediatrics.
Pregnancy: Gestational age, when applicable, is of extreme importance as the pharmacokinetics of the antiretroviral may vary according to the trimester of the pregnancy.
Race: Patients of different ethnic backgrounds may metabolize drugs at different rates. This could be explained by genetic polymorphisms of certain metabolic enzymes which can contribute to large interpatient pharmacokinetic variability of antiretrovirals.
Indication: The pharmacological advice may be influenced by the reason for which the TDM was requested.
All current medications: We require a list of prescribed medications, over the counter medications and natural health products taken by the patient. Possible interactions will be analyzed and taken into consideration in the interpretation and pharmacological advice provided.
History of virologic failure, resistance and adherence information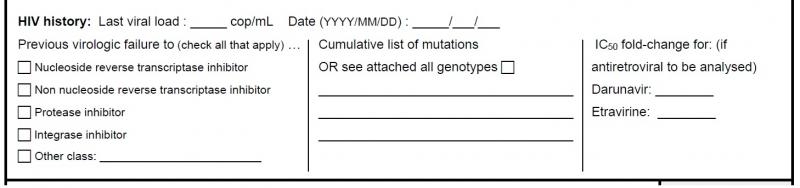
Previous virologic failure: The target values for patients with prior virologic failure to one or different classes of antiretrovirals may be higher in comparison to those without prior failure.
Cumulative list of mutations: The resistance data is incorporated into the interpretation of drug concentrations by means of the genotypic inhibitory quotient (GIQ) and virtual inhibitory quotient (vIQ). The physician may either transcribe the cumulative mutation list or fax a copy of all the genotypes done for a given patient.
IC50 fold-change: It is recommended to transcribe the fold-change of darunavir or etravirine if a virtual phenotype is available. If several virtual phentypes were done, please fax a copy of all results for a more accurate interpretation.
Procurement and dosing information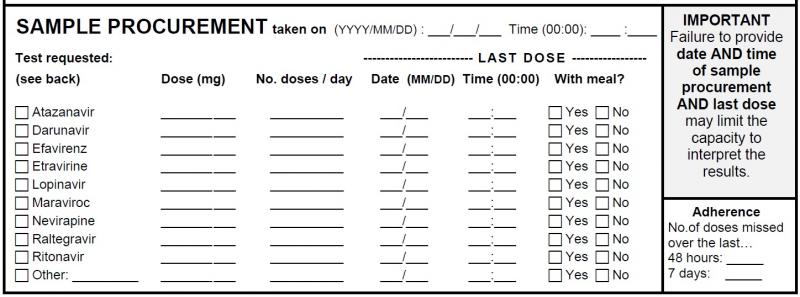
Procurement, date and time of last intake: The time and date of sample procurement and of the last dose intake is of paramount importance as plasma concentrations vary according to the time elapsed between the last dose and the procurement time. The same concentration could be supratherapeutic, therapeutic or subtherapeutic depending on when the procurement time was in relation to the last dose.
Antiretroviral(s) to analyze: The antiretroviral(s) to be analyzed must be checked off to receive a report. Note that you will not be charged for ritonavir TDM when it is accompanying a protease inhibitor as a pharmacoenhancer. Furthermore, you will not receive an interpretation report specifically for ritonavir. It is however important to provide the dose and number of doses per day of ritonavir as this may influence the pharmacokinetics of the active protease inhibitor.
Dose, # of doses/day: The concentrations of a drug will vary according to the dosing regimen. Also, dosing recommendations are made according to the patient’s current dosing regimen.
Intake with meal: The intake with or without food may greatly influence the absorption of certain antiretrovirals. This is also taken into account when making recommendations.
Antiretroviral adherence: The number of doses missed over the last week may influence the plasma concentration of the analyzed drug and must be taken into consideration in the interpretation of the result.
Pharmacokinetic interpretations of antiretrovirals are generally based on trough concentrations; that is the concentration at the end of the dosing interval. Therefore, for most antiretrovirals, blood should be drawn just prior to the next dose or as close as possible to the next dose.
If obtaining a trough concentration is not possible, blood drawing should be done as follows:
- For efavirenz:
More than 10 hours post dose
- For raltegravir in a patient with resistance to three classes of antiretroviral:
Any time in the dosing interval
- For other antiretrovirals administered once daily:
12-26 hours post dose
- For antiretrovirals administered twice daily:
6-14 hours post dose
If malabsorption is suspected, a sample can be taken at the time when the concentration is expected to be at its maximum (Tmax). The Tmax varies between the different antiretrovirals but is generally 2 to 4 hours post-dose.
Blood should be drawn into a sodium heparinized tube (non gel). One sample is sufficient even if more than one antiretroviral is to be measured.
Sample preparation and storage
The blood should be drawn in a heparinized (green top, non gel) tube. For adults, 1 mL of plasma per specimen in a cryotube (≥ 1.5mL size) is required. For children, a 200 mcL plasma specimen is sufficient. When several antiretrovirals are requested, one tube is sufficient.
The heparinized sample should be centrifuged (3000 x g for 5 minutes) within 6 hours of sample procurement. The plasma should be separated before sending it to the Québec Antiretroviral TDM Program Laboratory.
Plasma shipped the same day as it is drawn should be kept at 4ºC. Otherwise, the plasma should be frozen and stored at ≤ - 20ºC until it is sent.
Specimen Transport
The sample should be shipped to the Central Laboratory at the McGill University Health Centre (see address below) on Monday, Tuesday or Wednesday to ensure reception before the weekend.
Send the specimen at room temperature, in accordance with the guidelines for shipping infectious materials. If shipping requires 48 hours or less, no dry ice is required. However, if you foresee that shipping will exceed 48 hours, include dry ice in the shipping box and use appropriate safety measures for the dry ice.
Shipping Address 
Centralised Lab Reception, Room E05.3028
McGill University Health Centre
1001 Boulevard Décarie
Montréal (Québec)
H4A 3J1, Canada
Québec, Canada:
For samples originating from the province of Québec, the cost of antiretroviral plasma concentration analysis and interpretation is covered by the Ministère de la Santé et des Services sociaux du Québec. No cost will be charged to patients, healthcare professionals, clinics or institutions. The cost of specimen transport, however, must be covered by the site sending the sample to the program.
Outside Québec, Canada:
For samples originating from outside the province of Québec, the cost of antiretroviral plasma concentration analysis and interpretation is 47.50 $ CAD for the first antiretroviral measured and 27.50 $ CAD for any additional antiretrovirals measured from the same sample.
Of note, ritonavir concentrations are not usually reported when used to boost protease inhibitor concentrations and is therefore not charged.
We ask that you verify with your biochemistry and/or microbiology department if these tests are reimbursed by your hospital, province or state. Billing will be sent to the establishment sending the sample unless indicated otherwise. The cost for the specimen transport is to be covered by the sending institution. We do not accept COD orders.