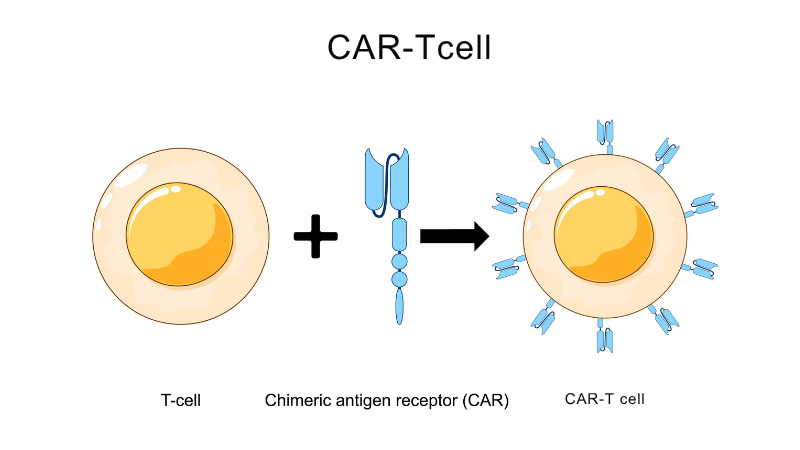
The MUHC CAR-T Cell Therapy Program offers new immunotherapies to treat cancer. The "CAR" in CAR-T is short for chimeric antigen receptor.
CAR-T cell therapy uses white blood cells (T lymphocytes) from the patient that are genetically modified to recognize and destroy certain types of cancer cells.
Our team includes specialized hematologists, nurses, laboratory technologists, and administrative assistants.
Which cancers can be treated using CAR-T cell therapies?
CAR-T cell therapies are approved and available at the MUHC for:
- relapsed/refractory diffuse large B-cell lymphoma
- relapsed/refractory B-cell acute lymphoblastic leukemia
- relapsed/refractory mantle cell lymphoma
- relapsed/refractory follicular lymphoma
CAR-T cell therapies may also be available in clinical trials for other health situations. If you have questions about these therapies or your eligibility, please speak to your treating physician.
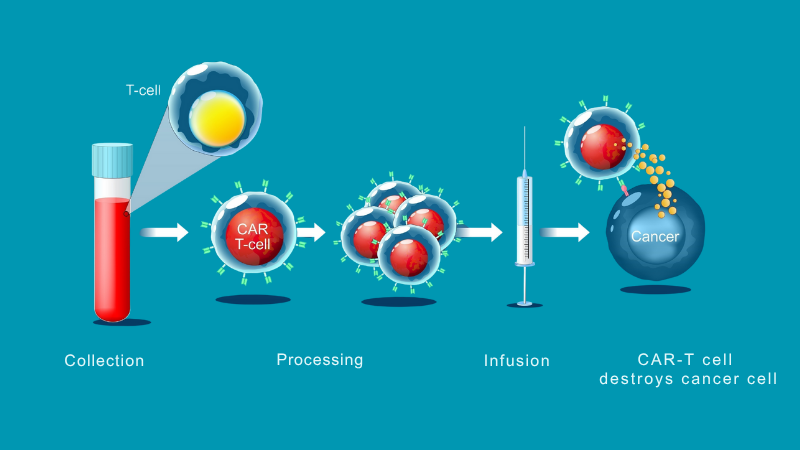
Steps in the CAR-T therapy process
Our CAR-T cell therapy team will meet with you to determine if you are eligible for this therapy.
You may have to come for several appointments, and more tests may be needed to confirm you are a good candidate for this treatment.
Some of your white blood cells (peripheral blood lymphocytes) will be collected.
This is done using a process called cellular apheresis, which uses a machine to separate and collect white blood cells from your blood.
The collected cells are sent to a specialized laboratory where the T cells are isolated, genetically modified to target your cancer, frozen and returned to the MUHC.
This process can take several weeks. During this time, the CAR-T cell therapy team may recommend extra therapies to control your cancer.
Your health and cancer status can change while waiting for the CAR-T cells to be ready, so our team will keep evaluating you to make sure CAR-T cell therapy is still right for you.
You will receive chemotherapy to prepare your body for the CAR-T cell therapy. It is usually done in the outpatient clinic.
About five days after the chemotherapy, you will receive your CAR-T cells.
This process is called infusion.
Infusion is a lot like a blood transfusion and takes about 30 minutes.
You will need to be monitored for side effects. This is mainly done while you are in hospital, but some of the monitoring might be done while you are at home or in an outpatient setting near the hospital.
During the first 30 days after treatment
You will have regular and frequent appointments at the MUHC with the CAR-T Cell Therapy team. You must always stay within one hour of the MUHC. You must have a caregiver with you 24 hours a day during this period and the CAR-T Cell coordinator must know who you have chosen to be your caregiver. If you live more than 60 minutes away from the hospital, we will arrange nearby housing. Financial assistance for travel and housing may be available.
During the first three months after treatment
You will come back to the MUHC for visits, blood tests and/or medical imaging to monitor how well the CAR-T cell therapy is working and to check for complications.