MUHC leads with first patients in Canada enrolled in global radioligand therapy trial for metastatic cancer
One year after becoming the first centre to open a global clinical trial of a radioligand therapy for metastatic prostate cancer, the MUHC is now offering a second trial of the same kind—this time for patients with other advanced cancers—marking another promising step forward in precision nuclear medicine.
Patients with advanced pancreatic, lung or breast cancer at the McGill University Health Centre (MUHC) are the first in Canada to test the safety and efficacy of a new radioligand cancer therapy. Unlike chemotherapy, immunotherapy or traditional radiation therapy, radioligand therapy (RTL) uses elements called ligands and radioisotopes to target and kill cancer cells, wherever they are in the body. The drug tested in this international clinical trial has shown significant antitumour activity in preclinical studies and produced positive results in the trial’s first phase.
In this phase 2 of the so-called LuMIERE clinical trial, sponsored by Novartis, the investigative drug—[177Lu] Lu FAP 2286—will be administered intravenously, either as a monotherapy in patients with pancreatic ductal adenocarcinoma (PDAC), non-small cell lung cancer (NSCLC) or breast cancer, or in combination with chemotherapy in patients with untreated PDAC or relapsed NSCLC.


“We are leading the charge in bringing radioligand therapy to Canadian cancer patients. This trial is a major milestone that reinforces our role as a national leader in nuclear precision medicine. Our goal is clear: to offer new hope and innovative experimental treatment options to patients who have exhausted conventional therapies,” says Dr. Ramy Saleh, medical oncologist at the MUHC’s Cedars Cancer Centre and Medical Director, Oncology Clinical Trials, at the Centre for Innovative Medicine (CIM) of the Research Institute of the MUHC (The Institute).
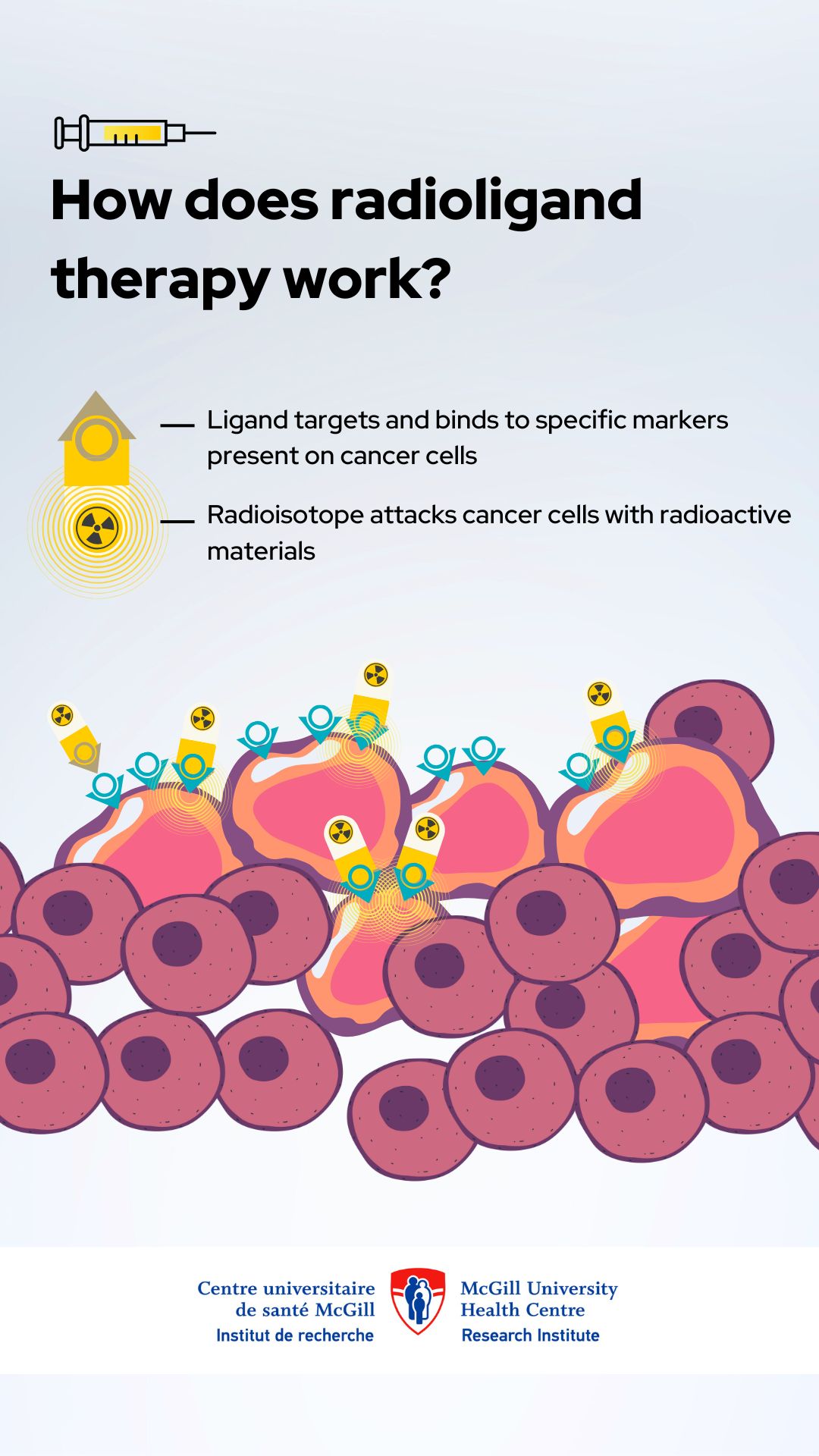
How does the therapy work?
The investigational treatment specifically targets fibroblast activating proteins (FAPs), which are produced by cancer-associated fibroblasts—groups of cells within the tumour microenvironment that interact with the tumour. And that’s where the potential of this therapy lies: by honing in on FAPs, it could enhance treatment effectiveness while sparing healthy tissue.
“This therapy is designed to zero in on FAPs, ensuring the radioactive treatment reaches cancer cells while sparing normal tissue. This level of precision could redefine cancer treatment as we know it,” explains Dr. Farzad Abbaspour, Head of the MUHC Nuclear Medicine Division within the Department of Medical Imaging. “To confirm the presence of FAPs, patients will undergo PET scan screening with a specialized imaging agent before starting treatment.”
“Radioligand therapy is an exciting new frontier in oncology, and at the MUHC, we are determined to be at the forefront of this revolution. Our commitment is to push the boundaries of cancer treatment and bring cutting-edge clinical trials to our patients here in Quebec and in Canada,” adds Dr. Saleh, also Investigator in The Institute’s Cancer Research Program and Assistant Professor in the Gerald Bronfman Department of Oncology at McGill University.
With this clinical trial, made possible through the collaboration of the MUHC’s medical oncology and nuclear medicine teams, and The Institute’s Centre for Innovative Medicine, the MUHC confirms its commitment to remaining at the forefront of cancer treatment trials, in order to offer the latest therapeutic innovations to Quebec patients and contribute to the advancement of science.
Media contact
Fabienne Landry
Communications coordinator, Research, MUHC
[email protected]